viewphii-US Product Information
Specification
| Product | Linear Model (Linear Probe + Tablet Set) |
Convex Model (Convex Probe + Tablet Set) |
|
|---|---|---|---|
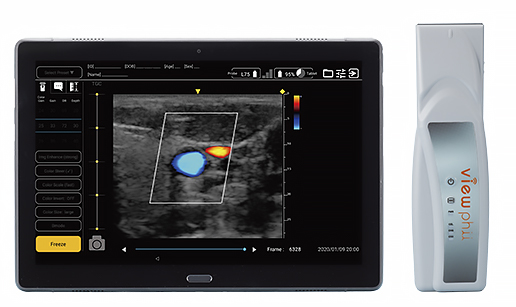
|
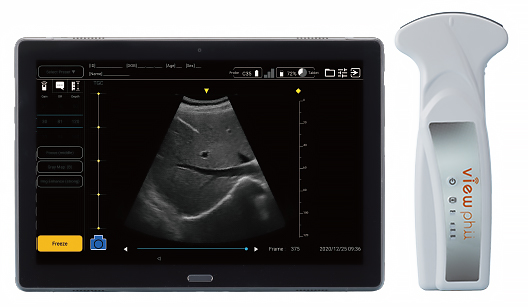
|
||
| Targeted sites | Orthopedic area (shoulder, hand, elbow, knee) Vascular areas (cervical, upper and lower limbs) Mammary gland, anesthesia, thyroid, lung, etc. |
General abdomen (liver, kidney, gallbladder, bladder abdominal aorta, obstetrics and gynecology), lung Anesthesia (nerve block), etc. |
|
| Product Part No. | SC0H02A-ALVP1 | SC0H02A-ACVP1 | |
| Product No. | VP-US L75-01 | VP-US C35-01 | |
| JAN code | 4580337438029 | 4580337438036 | |
| Generic name | Ultrasound diagnostic system | ||
| Brand name | viewphii-US series | ||
| Approval No. * | 301ABBZX00039000 | ||
| Probe | Size (H x W x D) | Approx. 172 x 50 x 28mm | Approx. 180 x 72 (probe tip)/ 50 (handle) x 28mm |
| Weight | 165g | 185g | |
| Built-in battery | Lithium Battery | ||
| Battery operation time | More than 3 hours of continuous operation (with standard use) | ||
| Charging method | USB charging | ||
| Wireless | Wi-Fi (wireless connection), USB (wired connection) | ||
| Display mode | B mode, Color Doppler | B mode | |
| Measurement area (W x D) | Approx. 34mm x 80mm | Approx. 54mm x 200mm | |
| Tablet | 10-inch LCD, Android OS | ||
| Product line-up | Main unit (tablet), probe, probe cable, charger for probe, charger for main unit, USB cable for main unit | ||
*The Japanese Pharmaceutical and Medical Device Act (PMD Act)
The exterior design and specifications are subject to change for improvement without notice.
Always read the package insert and operation manual before use.
Please contact us for support plans.
![Mobile Medical Solution [viewphii]](/en/common/images/viewphii_logo.png)
![Mobile Medical Solution [viewphii]](/en/common/images/viewphii_logo_sp.png)